Outpatient Clinical Research Unit
This service is provided by the Clinical Research Resource (CRR).
Overview
The Outpatient Clinical Research Unit on Presbyterian Hospital 10th Floor (PH-10) offers a highly personalized and professional environment for clinical investigators and adult and pediatric research participants. Nearly 6,000 research visits from more than 100 protocols take place in the Outpatient Clinical Research Unit each year. The Outpatient Clinical Research Unit features:
- Eight fully-equipped physical examination rooms
- Dental operatory
- Four interview/consultation rooms
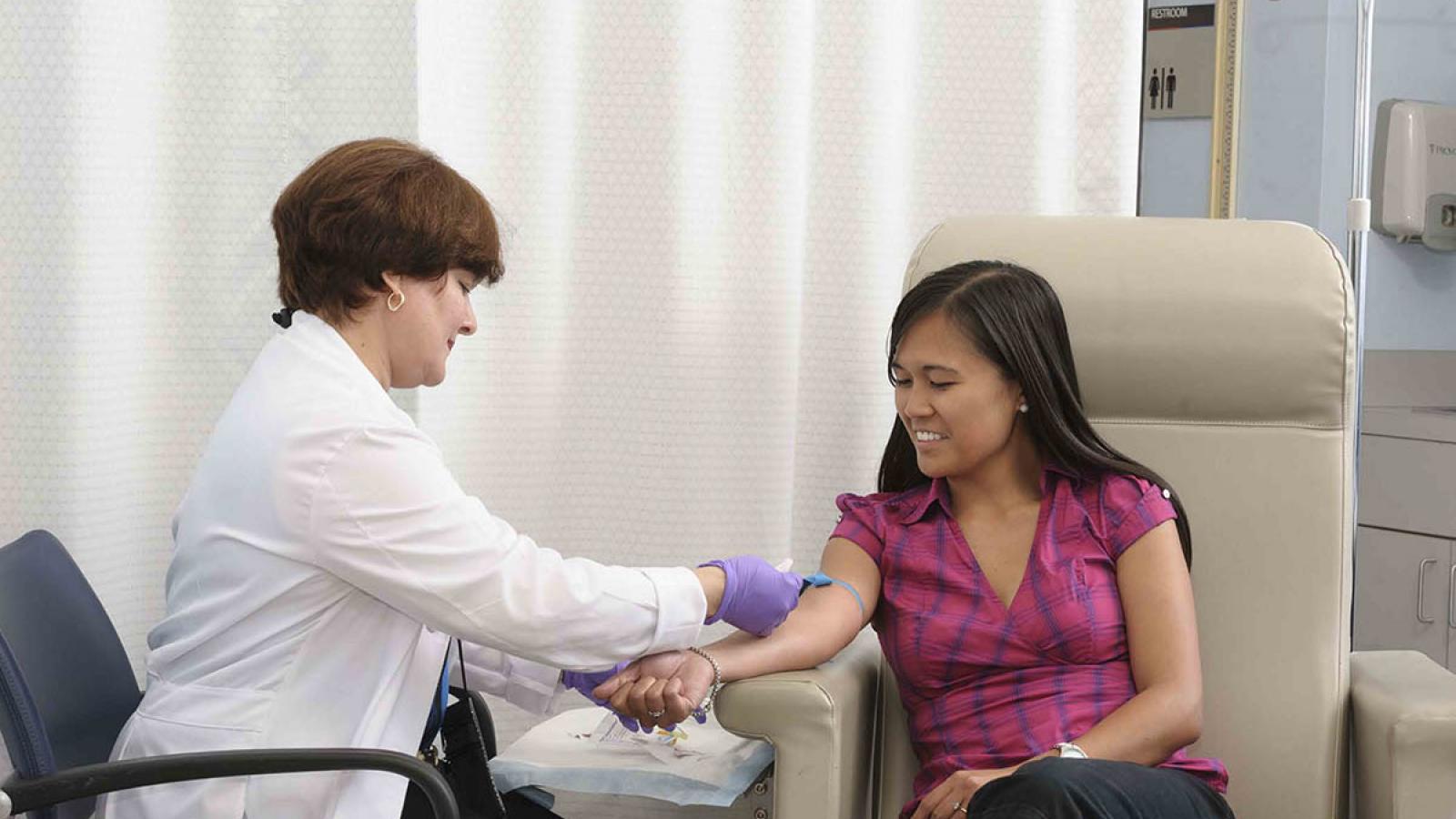
- Phlebotomy station
- Specimen processing area (includes two refrigerated centrifuges)
- Reception area and participant waiting room
- Limited nursing assistance, skilled phlebotomy services, some specimen processing, EKGs, and vital signs
Eligibility
This service is available to all researchers at Columbia University.
- Protocols must be IRB and CRR approved (simultaneous submission to the CRR can be completed)
- Budget must include all applicable CRR fees
- Available medical coverage (MD, NP, or PA) during patient visits
- Principal investigators must ensure that trained staff, with protocol specific competencies, are available to complete protocol needs.
- Ability to provide specific equipment to complete protocol requirements
- Study team members responsible for scheduling appointments in the Clinical Research Resource Outpatient Unit must complete a mandatory training. Please contact us for more information on the IMPACT training schedule.
For all relevant documents and guidelines, click here
Hours
Monday-Friday
7:30 AM - 5:00 PM
Additional hours and Saturdays available by arrangement.
The Integrated Model for Patient Care and Clinical Trials (IMPACT) scheduling system can be used to book space in the Outpatient Clinical Research Units.
Costs
Note: NIH K awards are granted a $5,000 annual subsidy, capped at $25K per protocol.
|
Service |
FY26 Industry Initiated Studies* Charged by NYPH Directly |
FY26 Prices for all studies, regardless of funding* |
|---|---|---|
|
Outpatient Exam/Interview Room per hour (billed in 15 min. intervals) |
$29.82 |
|
|
Outpatient Blood Draw (per needle stick) |
$18.24 |
|
|
Outpatient Blood Processing (per participant) Simple (<15 min.): Basic (16-30 min.): Advanced (31-60 min.): Complex (>60 min.): |
$22.58 $33.87 $67.74 $112.90 |
|
|
Vitals (Height, Weight, HR, BP & RR) |
$18.24 |
|
|
EKG (Interpretation not included) |
$22.58 |
|
|
Outpatient Nursing Assistance per hour* (billed in 15 min. intervals) *Hourly rate includes salary plus fringe. Limited to specific services including: Participant Education, Physical Exams, and Pregnancy Tests. Protocol specific nursing services will only be provided if agreed upon as a necessity, during the protocol review process. |
$115.76 |
|
|
Startup Fee (in effect from 01/01/2024) |
$2,921.63 per protocol |
|
*All prices include salary plus fringe. No additional charges will be applied. Prices are reviewed on an annual basis and are subject to change. Studies will be charged the price in place at the time service is rendered.
For FY26 and beyond pricing on a future project, please look here.
To request a Project Cost Estimate (CE) on a non-industry funded study, please contact Associate Director for Finance & Administration, Ms. Helena Rincón, hr2016@cumc.columbia.edu, at the Irving Institute for Clinical and Translational Research.
Cite it, Submit it, Share it!
If your research has benefited from one or more Irving Institute resources, please remember to:
- Cite our CTSA grant, UL1 TR001873, in any relevant publications, abstracts, chapters, and/or posters.
- Submit your publications to PubMed Central (PMC) for compliance with the NIH Public Access Policy.
- Share your research updates with us by sending an email to: irving_institute@cumc.columbia.edu